pH stability in mixtures with less peat
More and more I am being asked how it is possible that the pH fluctuates so much more during the culture than it used to.
The right pH in the substrate is very important for optimal absorption of the various minerals.
This starts on day 1, so potting soils and substrates must have the correct pH before use.
First the theory:
The pH stands for the negative logarithm of the concentration of H+ ions (which we measure in the free available water).
The pH which is desired in horticulture is between 4 and 6.5, with 5.7-6.2 for most crops.
At this pH, the elements can be optimally absorbed by the plant.
Peat has a good buffer capacity and therefore the changes will not be too abrupt.
This is much less the case for other raw materials such as wood fiber and bark, as well as coco, and the pH will therefore be less stable in those substrates.
Often the pH must be increased. The need for lime differs per raw material. For example, peat has a lower pH value than coconut or wood fiber and will therefore need more lime.
Lime is added to raise the pH value (CaCO3 + often MgCO3).
The CO3 reacts with 2 H+ ions and together forms H2CO3 (which later gives H2O and CO2). By adding the CO3–, 2 H+ ions have been captured and thus the pH increases.
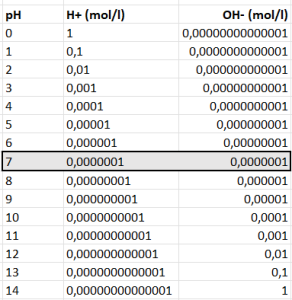 As you can see in the table, the lower the pH, the more work to raise (from 5.5 to 6 is easier than from 4.5 to 5).
As you can see in the table, the lower the pH, the more work to raise (from 5.5 to 6 is easier than from 4.5 to 5).
There are several ways to increase the pH, for example, potassium bicarbonate can also be used (KHCO3) or with KOH.
An additional reason to add dolomite lime is the Calcium and Magnesium that are dosed directly.
We therefore see that regular monitoring of the pH in the substrate becomes increasingly necessary as less peat and more other raw materials are used.
Independent Substrate Consulting (substrate-consulting.com)


 Previous Post
Previous Post Next Post
Next Post